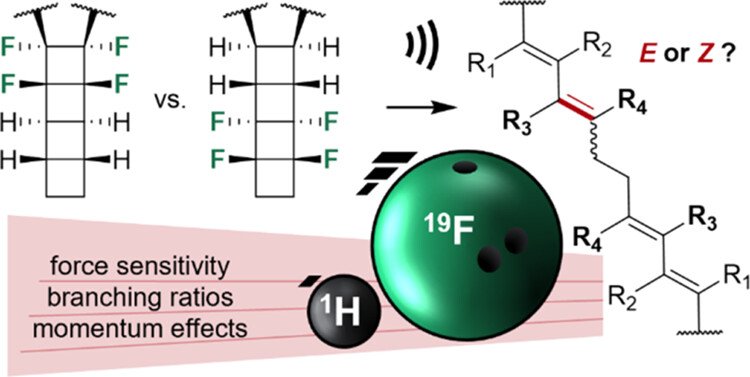
Matías Horst, Søren Holm, Leoš Valenta, Tatiana B. Kouznetsova, Jinghui Yang, Noah Z. Burns, Stephen L. Craig, Todd J. Martínez, Yan Xia, J. Am. Chem. Soc., 2024, ASAP Articles
Structure–Function Relationships in Pure Archaeal Bipolar Tetraether Lipids
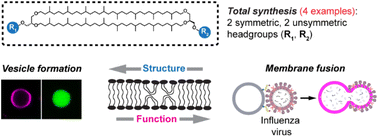
Ahanjit Bhattacharya, Isaac D. Falk, Frank R. Moss III, Thomas M. Weiss, Khoi N. Tran, Noah Z. Burns, Steven G. Boxer, Chem. Sci. 2024, 15, 14273–14286.
Yanan Li, Ting Yu, Xi Feng, Bo Zhao, Huahui Chen, Huan Yang, Xing Chen, Xiao-Hua Zhang, Hayden R. Anderson, Noah Z. Burns, Fuxing Zeng, Lizhi Tao, Zhirui Zeng, Nat. Commun. 2024, 15, 5256
Total Synthesis of (+)-Discorhabdin V
Brandon C. Derstine, Alina J. Cook, James D. Collings, Joseph Gair, Josep Saurí, Eugene E. Kwan, Noah Z. Burns, Angew. Chem. Int. Ed. 2024, 63, e202315284. ChemRxiv (2023), Synfacts
2023
A Metal-Free Cyclobutadiene Reagent for Intermolecular [4 + 2] Cycloadditions
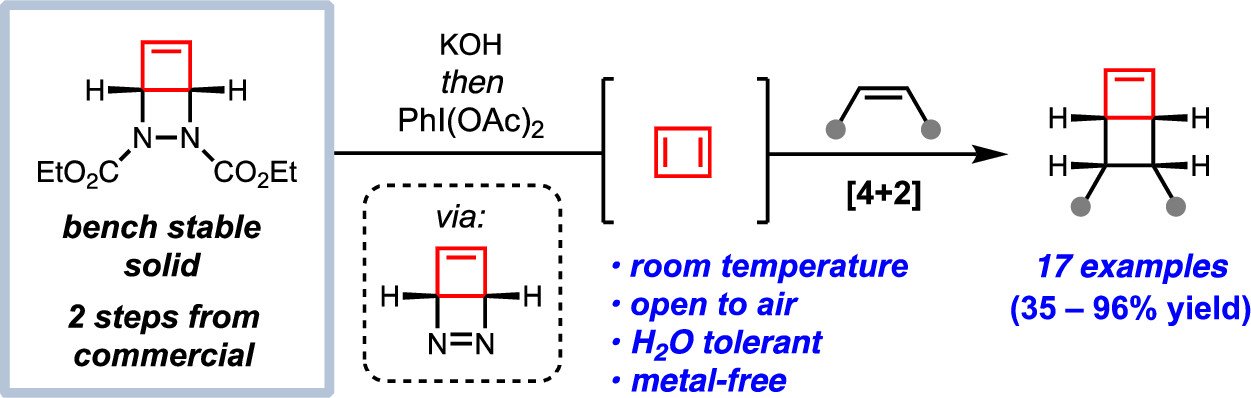
Benjamin R. Boswell, Carl M. F. Mansson, Gabrielle E. Cabrera, Calvin R. Hansen, Allen G. Oliver, Noah Z. Burns J. Am. Chem. Soc. 2023, 145, 5631–5636.
2022
Aqueous Amine-Tolerant [2+2] Photocycloadditions of Unactivated Olefins
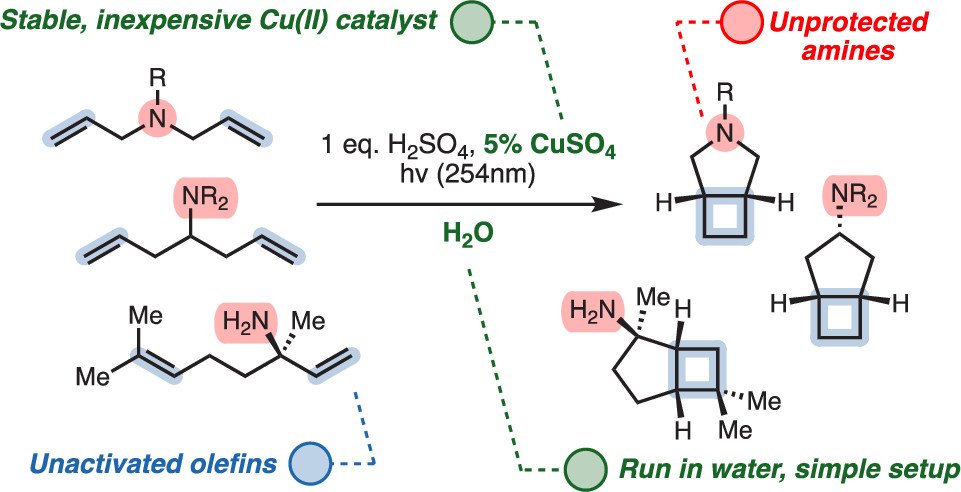
Carl M. F. Månsson and Noah Z. Burns, J. Am. Chem. Soc. 2022, 144, 19689–19694. Synfacts, OPRD Highlights
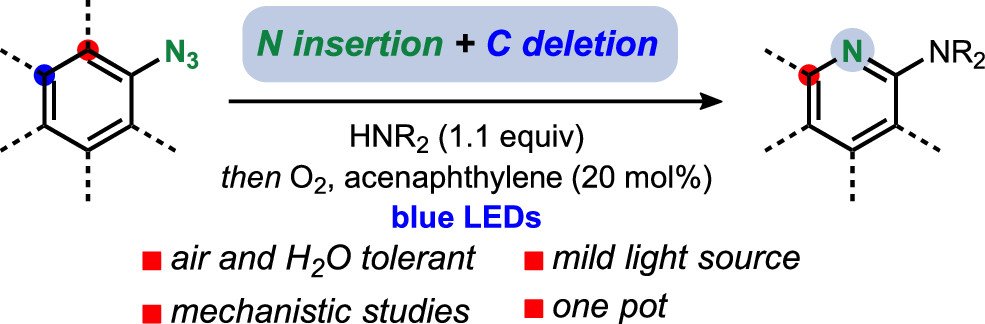
Conversion of Aryl Azides to Aminopyridines
Sajan C. Patel and Noah Z. Burns, J. Am. Chem. Soc. 2022, 144, 17797–17802. ChemistryViews, Synfacts
Gabrielle E. Cabrera, Tristen A. Reid, Eric C. Johnson, Joshua A. Orlicki, Noah Z. Burns, Jesse J. Sabatini. ChemPlusChem 2022, 87, e202200096
Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation
Tzu-Tang Wei, Mark Chandy, Masataka Nishiga, Angela Zhang, Kaavya Krishna Kumar, Dilip Thomas, Amit Manhas, Siyeon Rhee, Johanne Marie Justesen, Ian Y. Chen, Hung-Ta Wo, Saereh Khanamiri, Johnson Y. Yang, Frederick J.Seidl, Noah Z. Burns, Chun Liu, Nazish Sayed, Jiun-Jie Shie, Joseph C. Wu. Cell 2022, 185 (10), 1676–1693
2021
Sajan C. Patel, Myles W. Smith, Jaron A. M. Mercer, Kensuke Suzuki, and Noah Z. Burns. Org. Lett. 2021, 23, 6530–6535
Enantioselective Total Synthesis of the Archaeal Lipid Parallel GDGT-0 (Isocaldarchaeol)
Isaac D. Falk, Bálint Gál, Ahanjit Bhattacharya, Jeremy H. Wei, Paula V. Welander, Steven G. Boxer, and Noah Z. Burns*. Angew. Chem. Int. Ed. 2021, 60, 17491–17496
Jingbai Li, Patrick Reiser, Benjamin R. Boswell, André Eberhard, Noah Z. Burns*, Pascal Friederich*, and Steven A. Lopez*. Chem. Sci. 2021, 12, 5302
Mechanochemical Synthesis of an Elusive Fluorinated Polyacetylene
Benjamin R. Boswell, Carl M. F. Mansson, Jordan M. Cox, Zexin Jin, Joseph A. H. Romaniuk, Kurt P. Lindquist, Lynette Cegelski, Yan Xia*, Steven A. Lopez*, Noah Z. Burns*. Nat. Chem. 2021, 13, 41–46.
2020
Halogenation-Dependent Effects of the Chlorosulfolipids of Ochromonas danica on Lipid Bilayers
Frank R. Moss III, Gabrielle E. Cabrera, Grace M. McKenna, Giulio J. Salerno, Steven R. Shuken, Matthew L. Landry, Thomas M. Weiss, Noah Z. Burns*, and Steven G. Boxer*. ACS Chem. Bio. 2020, 15 (11), 2986–2995
Jinghui Yang, Matias Horst, Sabrina H. Werby, Lynette Cegelski*, Noah Z. Burns*, and Yan Xia*. J. Am. Chem. Soc. 2020, 142, 34, 14619–14626
The cascade unzipping of ladderane reveals dynamic effects in mechanochemistry
Zhixing Chen, Xiaolei Zhu, Jinghui Yang, Jaron A. M. Mercer, Noah Z. Burns*, Todd J. Martinez*. Nat. Chem. 2020, 12, 302–309
2019
“A convenient C–H functionalization platform for pyrroloiminoquinone alkaloid synthesis” Myles W. Smith, Isaac D. Falk, Hideya Ikemoto, and Noah Z. Burns. Tetrahedron. 2019, 75, 3366-3370. Article. Supporting Information. [Invited contribution in honor of Professor Ryan Shenvi, recipient of the 2019 Tetrahedron Young Investigator Award.]
"Enantioselective Synthesis of Azamerone” Matthew L. Landry, Grace M. McKenna, and Noah Z. Burns. J. Am. Chem. Soc. 2019, 141, 2867–2871. Article. Supporting Information. Synfacts.
2018
"Canvass: A Crowd-Sourced, Natural-Product Screening Library for Exploring Biological Space" Matthew D. Hall, Jason M. Rhode et al. ACS Cent. Sci. 2018, ASAP. Article. Supporting Information.
"Catalytic Regio- and Enantioselective Haloazidation of Allylic Alcohols" Frederick J. Seidl, Chang Min, Jovan A. Lopez and Noah Z. Burns. J. Am. Chem. Soc. 2018, 140, 15646-15650. Article. Supporting Information.
"Synthesis and Mechanochemical Activation of Ladderene–Norbornene Block Copolymers" Jessica K. Su, John D. Feist, Jinghui Yang, Jaron A. M. Mercer, Joseph A. H. Romaniuk, Zhixing Chen, Lynette Cegelski, Noah Z. Burns, and Yan Xia. J. Am. Chem. Soc. 2018, 140, 12388-12391. Article. Supporting Information.
"Ladderane phospholipids form a densely packed membrane with normal hydrazine and anomalously low proton/hydroxide permeability" Frank R. Moss III, Steven R. Shuken, Jaron A. M. Mercer, Carolyn M. Cohen, Thomas. M. Weiss, Steven G. Boxer, and Noah Z. Burns. Proc. Natl. Acad. Sci. 2018, 115, 9098-9103. Article. Supporting Information.
"Catalytic Enantioselective Dihalogenation in Total Synthesis" Matthew L. Landry, and Noah Z. Burns. Acc. Chem. Res. 2018, 51, 1260–1271. Article.
"Site-selective bromination of sp3 C–H bonds" Shyam Sathyamoorthi, Shibdas Banerjee, Justin Du Bois, Noah Z. Burns, and Richard N. Zare. Chem. Sci. 2018, 9, 100–104. Article. Supporting Information.
2017
"Enantiospecific Solvolytic Functionalization of Bromochlorides" Alexander J. Burckle, Bálint Gál, Frederick J. Seidl, Vasil H. Vasilev, and Noah Z. Burns. J. Am. Chem. Soc. 2017, 139, 13562–13569. Article. Supporting Information.
"Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene" Zhixing Chen, Jaron A. M. Mercer, Xiaolei Zhu, Joseph A. H. Romaniuk, Raphael Pfattner, Lynette Cegelski, Todd J. Martinez, Noah Z. Burns, and Yan Xia. Science 2017, 357, 475–479. Article. Supporting Information.
2016
"Chemical synthesis and self-assembly of a ladderane phospholipid" Jaron A. M. Mercer, Carolyn M. Cohen, Steven R. Shuken, Anna M. Wagner, Myles W. Smith, Frank R. Moss III, Matthew D. Smith, Riku Vahala, Alejandro Gonzalez-Martinez, Steven G. Boxer, and Noah Z. Burns. J. Am. Chem. Soc. 2016, 138, 15845–15848. Article. Supporting Information. ACS Select. Synfacts.
"Chiral Alkyl Halides: Underexplored Motifs in Medicine" Bálint Gál, Cyril Bucher, Noah Z. Burns. Mar. Drugs 2016, 14, 206. Article. [Invited contribution for Special Issue on Marine Organohalides]
"A Unified Approach for the Enantioselective Synthesis of the Brominated Chamigrene Sesquiterpenes" Alexander J. Burckle, Vasil H. Vasilev, Noah Z. Burns. Angew. Chem. Int. Ed. 2016, 55, 11476–11479. Article. Supporting Information. Synfacts.
"Selective Bromochlorination of a Homoallylic Alcohol for the Total Synthesis of (–)-Anverene" Frederick J. Seidl, Noah Z. Burns. Beilstein J. Org. Chem. 2016, 12, 1361–1365. Article. Supporting Information. [Invited contribution for Special Themed Issue on Strategies in Asymmetric Catalysis]
"Catalytic Enantioselective Dihalogenation and the Selective Synthesis of (–)-Deschloromytilipin A and (–)-Danicalipin A" Matthew L. Landry, Dennis X. Hu, Grace M. McKenna, Noah Z. Burns. J. Am. Chem. Soc. 2016, 138, 5150–5158. Article. Supporting information.
2015
"Natural products: Emulation illuminates biosynthesis" Jaron A. M. Mercer, Noah Z. Burns. Nature Chem. 2015, 7, 860–861.
"Highly Selective Synthesis of Halomon, Plocamenone, and Isoplocamenone" Cyril Bucher, Richard M. Deans, Noah Z. Burns. J. Am. Chem. Soc. 2015, 137, 12784–12787. Article. Supporting information.
"Catalytic Chemo-, Regio-, and Enantioselective Bromochlorination of Allylic Alcohols" Dennis X. Hu, Fritz J. Seidl, Cyril Bucher, Noah Z. Burns. J. Am. Chem. Soc. 2015, 137, 3795–3798. Article. Supporting information.
2013
"Catalytic Enantioselective Dibromination of Allylic Alcohols" Dennis X. Hu, Grant M. Shibuya, Noah Z. Burns. J. Am. Chem. Soc. 2013, 135, 12960–12963. Article. Supporting information.
Postdoctoral
Burns, N. Z.; Jacobsen, E. N. "Catalysis in Tight Spaces," Nature, 2012, 483, 278–279.
Burns, N. Z.; Witten, M. W.; Jacobsen, E. N. "Dual Catalysis in Enantioselective Oxidopyrylium-Based [5 + 2] Cycloadditions," J. Am. Chem. Soc. 2011, 133, 14578–14581.
Burns, N. Z.; Jacobsen, E. N. "Mannich Reaction," in Science of Synthesis, Stereoselective Synthesis, Vol. 2, De Vries, J. G.; Molander, G. A.; Evans, P. A., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2011; 785–834.
Graduate
Sella, E.; Weinstain, R.; Erez, R.; Burns, N. Z.; Baran, P. S.; Shabat, D "Sulfhydryl-Based Dendritic Chain Reaction," Chem. Commun. 2010, 46, 6575–6577.
Burns, N. Z.; Krylova, I. N.; Hannoush, R. N.; Baran, P. S. "Scalable Total Synthesis and Biological Evaluation of Haouamine A and Its Atropisomer," J. Am. Chem. Soc. 2009, 131, 9172–9173.
Burns, N. Z.; Baran, P. S.; Hoffmann, R. W. "Redox Economy in Organic Synthesis," Angew. Chem., Int. Ed. 2009, 48, 2854–2867.
Burns, N. Z.; Jessing, M.; Baran, P. S. "Total synthesis of Haouamine A: the Indeno-Tetrahydropyridine Core," Tetrahedron, 2009, 65, 6600–6610.
Burns, N. Z.; Baran, P. S. "On the Origin of the Haouamine Alkaloids," Angew. Chem., Int. Ed. 2008, 47, 205–208.
Baran, P. S.; Burns, N. Z. "Total Synthesis of (±)-Haouamine A," J. Am. Chem. Soc., 2006, 128, 3908–3909.
Undergraduate
Burns, N. Z.; Hackman, B. H.; Ng, P. Y.; Powelson, I. A.; Leighton, J. L. "The Enantioselective Allylation and Crotylation of Sterically Hindered and Functionalized Aryl Ketones: Convenient Access to Unusual Tertiary Carbinol Structures," Angew. Chem., Int. Ed. 2006, 45, 3811–3813.






















!["Chiral Alkyl Halides: Underexplored Motifs in Medicine" Bálint Gál, Cyril Bucher, Noah Z. Burns. Mar. Drugs 2016, 14, 206. Article. [Invited contribution for Special Issue on Marine Organohalides]](https://images.squarespace-cdn.com/content/v1/5c4b7fd670e802450d0e2f73/1551377811973-IHGY5A1KJXYOR1TMK2TL/2016+Chiral+Alkyl+Halides_MD.png)





